Lead Citrate, Trihydrate
*** Only available for customers in Germany ***
Lead citrate is used as enhancer for heavy metal staining in electron microscopy. It binds to osmium and uranyl acetate and thereby enhances the contrast of several cellular structures. Due to its high reactivity with CO2 care has to be taken for the preparation of solutions and during usage.
Permanently reduced price!
Product Details
Description
Specifications
| Minimum assay | 99% |
| Formula | Pb(C6H5O7)2·3H2O |
| Molecular weight | 1053.82 |
| CAS-# | 512-26-5 |
Technical Tips
Handling of Lead Solutions
Prepare and store lead solution in plastics rather than glass vials. Avoid breathing on sections during lead staining in order to maintain a CO2-free environment. Lead and CO2 form in the presence of Oxygen PbCO3 a white toxic precipitate.
Lead stains are very sensitive and will precipitate quickly upon contact with CO2. To prevent precipitation during preparation, storage or staining the following steps should be followed:
Preparation of CO2 free Water
Prepare CO2 free water: boil double distilled water (aqua bidest) and store the hot water in tightly sealed or glass stoppered bottles. Upon opening of these bottles, a hissing sound should appear indicating a proper seal. Only open the water bottle prior to use. Repeat the above procedure with an almost full bottle that was left open for longer than 10min.
Construction of an CO2-free Environment (Lead Staining Apparatus)
- Place a filter paper wetted with 1N NaOH in a petri dish with some NaOH pellets.
- Add a sheet of dental wax (Parafilm) on top of the wetted filter paper.
- The petri dish serves as the staining chamber. The NaOH will quickly absorb CO2 in the chamber, giving a CO2 free environment for lead staining.
References
- Book chapter for lead staining: Electron Microscopy Principles And Techniques For Biologists By John J. Bozzola And Lonnie D. Russell. 1992 Pp.115-118.
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Bio., 17, 208 (1963)
- Sato, T., J. Electron Micro., 116, 133 (1976)
- Venable, J.H. and Coggeshall, R. (1965). A simplified lead citrate stain for use in electron microscopy. J. Cell Biol., 25:407
- Famy, A. (1967). An extemporaneous lead citrate for electron microscopy. Proc. 25th Ann. EMSA Meet., p.148. Claitor's Pub. Division, Baton Rouge, LA.
More Information
| Application |
EM
|
|---|---|
| Signal word |
Danger
|
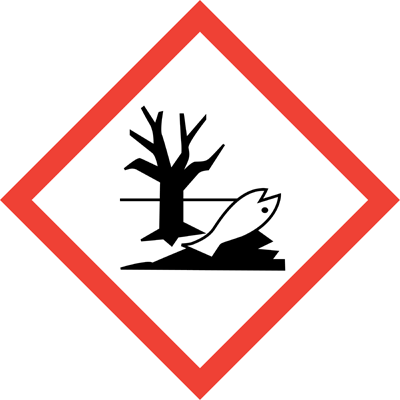
| Symbol GHS |
GHS07

GHS08

GHS09
|
| Hazard statements |
H302_H332
H360Df
H373
H410
|
| Precautionary statements |
P260
P281
P301_312
P304_340
P405
P501
|
| Condition |
solid
|
| Shipping Class |
Hazardous Good LQ
|
| Storage Temperature |
RT
|
| SDS (multilingual) | E17800, E17810 |