Array Tomography - Cut by Cut
 Connectomics – the mapping of neuronal pathways and connections of an organism’s nervous system – has been the driving force for the development of 3D-imaging techniques in light and electron microscopy.
Connectomics – the mapping of neuronal pathways and connections of an organism’s nervous system – has been the driving force for the development of 3D-imaging techniques in light and electron microscopy.
Due to resolution limits of light microscopy researchers started to investigate techniques capable of revealing the ultrastructure of neuronal systems to display neuronal connectivity. Thereby, three powerful techniques based on scanning electron microscopy (SEM) have been developed and tailored for volume imaging at nanometer resolution: Serial Block-Face Scanning EM (SBFSEM), Focused Ion Beam SEM (FIB-SEM) and Array Tomography (AT).
In comparison to SBFSEM and FIB-SEM, Array Tomography is a non-destructive technique that allows storage, re-imaging and re-staining of the obtained sections.
By now, Array Tomography is not only used in Connectomics but also in various other scientific areas e.g., cellular studies in cell biology or even the reconstruction of complex materials.
The Working Principle of Array
Tomography
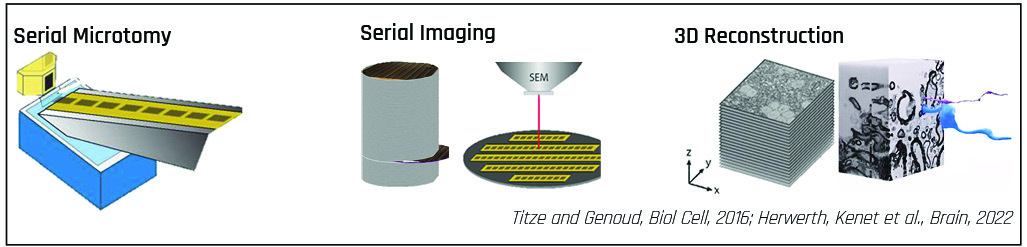
Samples are cut into series of sections with an ultramicrotome. The serial sections are collected on a substrate followed by SEM imaging and 3D-reconstruction. The scope of research defines the number of sections required for sample reconstruction.
The ATUMtome
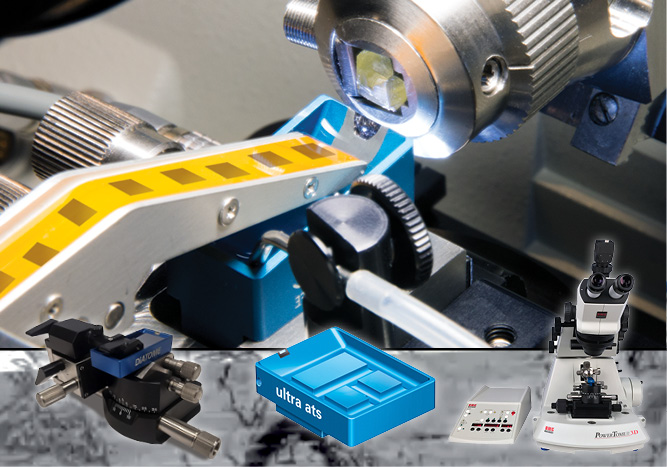
The ATUMtome is the Automated Tape-Collecting Ultramicrotome and part of the Array Tomography workflow. It has been developed by Richard Schalek and Jeff Lichtman at Harvard University.
This fully automated system collects sections cut with the ultramicrotome on a tape, which is immersed in water inside a diamond knife trough.
Key to a reliable performance is the precise communication between ultramicrotome and tape collector for fine-tuning the tape- and cutting-speed.
After sections have been collected on tape, the tape is cut into strips and mounted on a flat conductive substrate like a silicon wafer. The loaded wafer can be mounted into a SEM for image acquisition. If contrast enhancement is necessary, sections on wafers can be post-stained with heavy metals before SEM imaging or with fluorescent markers for CLEM workflows.
 ATUM Approaches
ATUM Approaches
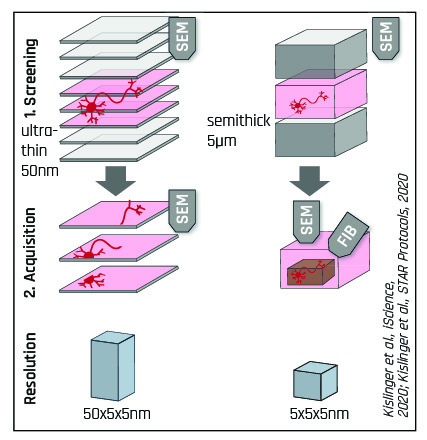
Apart from the standard ATUMtome approach, which is limited to a z-resolution of 30-50nm, a new hybrid method called ATUM-FIB has been developed that combines the advantages of both ATUMtome and FIB-SEM.
|
ATUMtome Features & Benefits Fully automated collection of <100 to >1.000 of sections Non-destructive: sections can be stored, re-imaged, re-stained Wide section thickness range: 30nm to 5µm Standard sample preparation techniques and resins usable |
Allows correlative microscopy for localization and then Multi-resolution and Multi-scale Imaging at different resolutions Less penalty for failure: Sample viability and complications are determined earlier in the process |




