GraFIX, a method for single particle cryo-electron microscopy (cryo-EM)
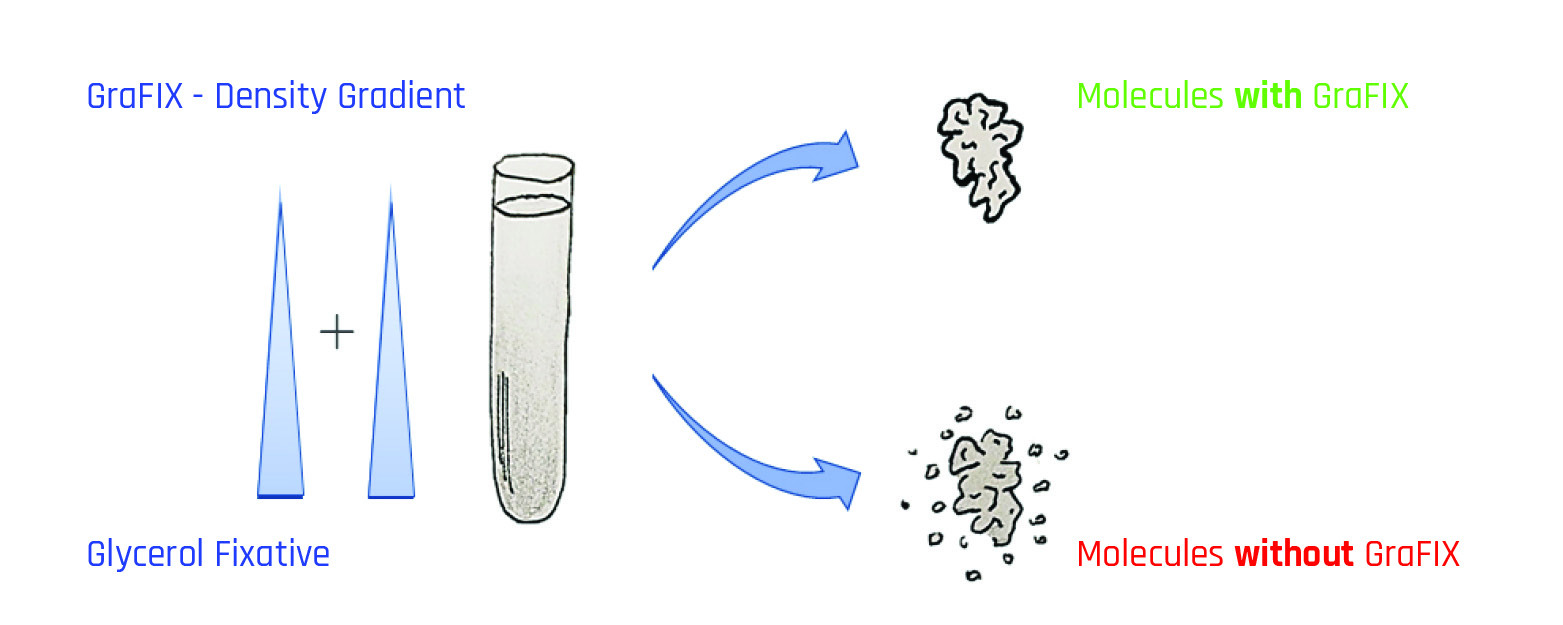
Fig. 1.: GraFIX method: The centrifugation tube contains a gradient of glycerol or sucrose and cross-linking reagent (e.g. glutaraldehyde or formaldehyde). Separation is based on sedimentation speed (rate zonal centrifugation). The GraFix treatment improves the properties and stability of the molecular complexes in contrast to the untreated sample, which shows degradation and destabilization artifacts.
For single particle structure determination in cryo-EM, purified proteins are frozen (vitrified) in their native state. However, the imaging contrast in cryo EM is very weak due to the low density of the elements of life. Negative staining can be used to increase contrast, but unstained native structural analysis is the method of choice. During macromolecule purification, sample heterogeneity also often occurs because of degradation processes and protein complexes are destabilized by in vitro buffer solutions. These and other complications can seriously compromise high-resolution 3D reconstruction.
The GraFix method (gradient fixation) can dramatically increase the quality and stability of proteins for single particle analysis (Kastner et al., 2008; Stark et al. 2010). Usually, macromolecules are purified and concentrated by density gradients. This is exactly where the GraFix method steps in. During rate-zonal density gradient ultracentrifugation, the macromolecules are exposed to a low-dose chemical crosslinking agent (fixative). This is added directly during the preparation of the gradient (Fig. 1).
Density gradients can be prepared in a few minutes using the tilted-rotation method with a Gradient Master or a Gradient Station from BioComp Instruments, Canada. Linear gradients with increasing concentration of e.g. glycerol or sucrose and an increasing content of fixative are produced. The molecules pass through this density gradient during ultracentrifugation, where they are separated based on their sedimentation speed. At the end of ultracentrifugation, bands of purified molecules that have undergone gentle fixation are formed. The concentrated sample can then be removed from the tubes, e.g. manually with a cannula. For higher purity and reproducibility, the sample can be collected with a BioComp Fractionator or a Gradient Station. The sample can be analyzed directly during fractionation by UV absorption or fluorescence emission with a TRIAX flow cell.
After fractionation, the individual particles can be additionally contrasted by negative staining or analyzed directly in cryo-EM after a short buffer exchange without staining.
Several studies have shown that the GraFix method significantly improves the imaging quality of single particle properties as well as molecular dispersion in cryo-EM (Stark et al. 2010; Wang et al. 2022). GraFix is highly reproducible and suitable for routine use.
Kastner B. et al. 2008, Nat Methods “GraFix: sample preparation for single-particle electron cryomicroscopy.”
Stark H. et al. 2010, Methods Enzymol “GraFix: stabilization of fragile macromolecular complexes for single particle cryo-EM.”
Wang L. et al. 2022, Nat Commun “Structure of nucleosome-bound human PBAF complex”









